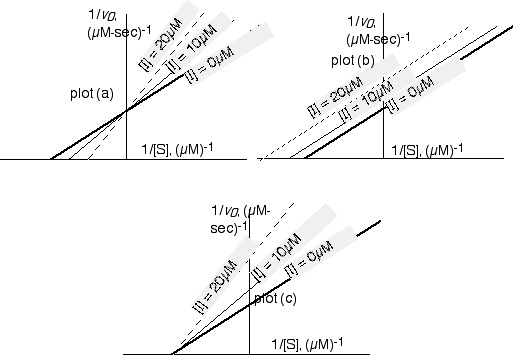
(a) If the inhibitor is a substrate analogue that binds in the enzyme's active site in the same manner as the substrate itself, which of these plots will illustrate the kinetic parameters of the reaction? What is the name for this type of inhibition? (3 pts)
The top-left plot illustrates this type of inhibition, which is competitive inhibition.
(1.5 points for knowing which plot is correct; 1.5 for knowing this is competitive.)
(b) If the inhibitor binds at a site separate from the active site, and the inhibition reversibly titrates either E or ES with inhibitor, effectively removing active enzyme molecules from solution, which plot will pertain? What is the name for this type of inhibition? (3 pts)
The bottommost plot illustrates this type of inhibition, which is noncompetitive.
(1.5 points for plot, 1.5 points for name)
No, it isn't. A protein is allosteric if its activity is modulated by the binding of another molecule. If the four active sites are independent, and there are no effector sites, then there is no allostery.
(2 points for knowing that this is not allosteric; 4 more for a plausible explanation. The wording doesn't have to be identical to mine.)
(a) Show that the slope of the Arrhenius plot is proportional to the activation energy ΔG‡. (3 points)
The relationship between rate constant k and activation energy ΔG‡ is given by
k = Q exp(-ΔG‡/RT)
So logk = log (Q exp(-ΔG‡/RT))
But the log of a product is the sum of the logs:
logk = logQ + log(exp(-ΔG‡/RT))
But log(x) = 2.303 ln x, so logk = logQ + 2.303 ln(exp(-ΔG‡/RT))
but ln(exp(z)) = z, so
logk = logQ + 2.303 *( -ΔG‡/RT)
i.e. logk = log Q + (-2.303 / R) * (ΔG‡) * (1/T)
which means that, in a plot of logk versus 1/T, the Y intercept will be log Q and the slope will be (-2.303/R) * (ΔG‡), which of course is proportional to ΔG‡.
(3 points for plausible explanation. If the distinction between natural log and common log is ignored, don't count off).
(b) Will the slope of the Arrhenius plot be steeper or flatter in an enzymatically-catalyzed reaction than in the corresponding uncatalyzed reaction? Explain. (3 points)
Flatter. What a catalyst does is reduce the activation energy, i.e. decrease ΔG‡. Therefore a catalyzed reaction has a smaller value of ΔG‡ and therefore the slope, (-2.303/R) * (ΔG‡), will be less negative. This corresponds to a flatter Arrhenius plot.
(1 point for recognizing it's flatter; 2 points for a good explanation.)
What I seek here is encapsulated in fig. 6.25 on pp. 190-191 of Horton. Not all the details need to be there, but for full credit there should be articulations of the nature of the E-S, E-TI1, Acyl-Enzyme, E-TI2, and E-P states, with drawings of at least half of them. The roles of the three required amino acids should be articulated at least as well as they were in the help-sheet, viz.
- Ser-195: forms tetrahedral intermediate with transition state)
- His-57: acid catalyst; proton donor / acceptor)
- Asp-102: stabilizes positive charge on His-57
(4 points for good diagrams, 5 points for good descriptions. Unusually good diagrams can count for more than four points if they make the English unnecessary).
B vitamin |
Compound built up of 5-carbon building blocks,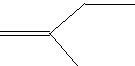 Isoprenoid Isoprenoid |
| Vitamin | Non-amino-acid component of a functional protein Cofactor |
| Coenzyme | Necessary nutrient that cannot be synthesized in the organism and must therefore be derived as is from the diet. Vitamin |
| Isoprenoid | Organic compound that functions as a non-amino-acid component of a functional protein, bound to the polypeptide portion throughout the course of the reaction. Prosthetic group |
| Prosthetic group |
Organic compound that functions as a non-amino-acid component of a functional protein Coenzyme |
| Cofactor | Water-soluble nutrient that cannot be synthesized in the organism; it serves as a coenzyme or as a precursor for a coenzyme. B vitamin |
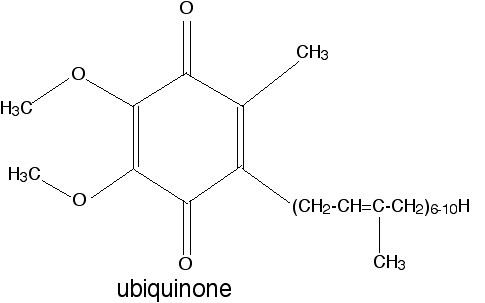
The relevant property is the relative stability of the semiquinone free radical that forms upon one-electron reduction of this compound.
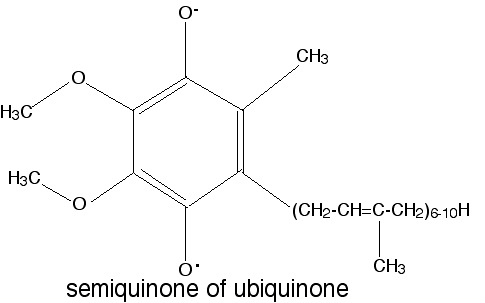
(4 points for a good explanation; 1 point for getting the actual name right. Drawing the semiquinone structure is not required, but a correct drawing can make up for a sloppy explanation).
Four. There are two chiral centers, at carbons 3 and 4:
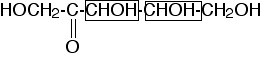
shown in the boxes in this drawing. Each chiral center contributes a factor of two to the count of stereoisomers (except in the case of meso compounds), so the total number of stereoisomers is 22 = 4.
(two points for knowing there are four stereoisomers; two points for a good explanation).
Cellulose
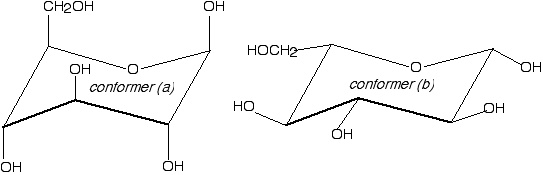
Conformer (b). All the bulky groups in conformer (b) are equatorial, so they don't sterically hinder one another nearly as much as the groups in conformer (a).
(1 point for getting the correct conformer, 2 points for a plausible explanation).
The group that is present in glucose and lcatos is a free aldehyde group HC=O that can be oxidized to an carboxylic acid group if exposed to a mild oxidant like Cu2+.
(1.5 points for knowing that the reactive group is the free aldehyde; 1.5 point for knowing that it is converted to a carboxylic acid. Give only 1 out of 1.5 for the second part if the word "carboxlic" is omitted.
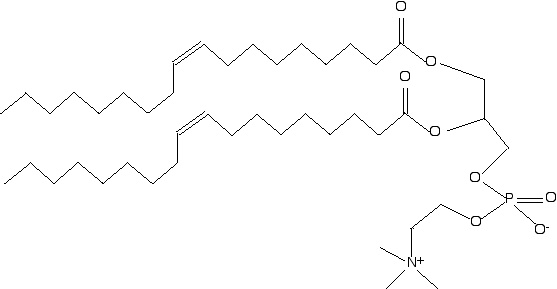
I would modify the fatty acyl group attached to the C1 carbon of glycerol from an oleate group to a saturated fatty acyl group like palmitate or stearate; the most common phospholipids have a saturated fatty acid attached at C-1 and an unsaturated fatty acid at C-2.
(2 points for knowing that the appopriate change would be to replace an unsaturated FA with a saturated FA; 2 more points for knowing that phospholipids generally have a saturated FA at C-1 and an unsaturated FA at C-2.)
(a) Would we expect to find any dye on the southeast corner of the outer surface of the bilayer? Why? (2 pts)
Yes: there is rapid diffusion within a surface of a bilayer, and 5 sec is sufficient to carry the dye across the diagonal of a cell.
(1 point for saying yes, 1 point for understanding that it involves rapid diffusion within the layer.)
(b) Would we expect to find any dye on the northwest corner of the inner surface of the bilayer? Why? (2 pts)
No: Diffusion from one side of the bilayer is extremely slow, except in the presence of flippase enzymes.
(1 point for saying No; 1 point for a good explanation. The part about flippase enzymes is unnecessary).
| Reaction | ΔG | Reaction | ΔG | Reaction | ΔG | ||
| 1 | -35 | 2 | -2 | 3 | -3 | ||
| 4 | -27 | 5 | -4 | 6 | 0 | ||
| 7 | -1 | 8 | -29 |
The irreversible reactions are reactions 1, 4, and 8. We know that because they are the reactions for which ΔG is large and negative; thus the reverse reaction is negligible.
(1 point for knowing 1, 4, and 8 are the irreversible ones; 1 point for recognizing that reactions with ΔG well below zero are irreversible or nearly so.) (b) Which reactions are likely to be control points in the pathway? Why? (2 pts)
Again, reactions 1, 4, 8. Control tends to be inserted at the irreversible steps, whereas product and substrate concentrations dominate the stabilization at the highly reversible steps.
(1 point for knowing that it's 1, 4, and 8; one point for articulating the idea that control is usually exerted at the irreversible steps.)
(c) The ΔGo' value for reaction 4 is -18 kJ/mol. This reaction is M + N -> O + P
The cellular concentrations of M, N, and O are 10 μM, 20 μM, and 5 μM respectively.
Calculate [P].(5 pts)
N.B.: In class I said that the temperature was 26.84° C,
and I reminded the class that the gas constant R = 8.314 J/(mole-deg)
We use the relationship between standard free energy and actual free energy:
ΔG = ΔGo' + RT ln Q,
and for a bimolecular reaction
Q = [O][P] / ([M][N])
Therefore
(ΔG - ΔGo') / RT = ln ([O][P] / [M][N])
So if we raise e to the power of each side of the equation,
exp[(ΔG - ΔGo') / RT] = ([O]/[M][N]) * [P], or
[P] = exp[(ΔG - ΔGo') / RT] * [M] [N] / [O]
We have said ΔG = -27 kJ/mol, ΔGo' = -18 kJ/mol, and that [M] = 10μM, [N] = 20μM, and [O] = 5μM. Thus, since T = 273.16 + 26.84°C = 300K
[P] = exp[(-27 kJ/mol + 18 kJ/mol) / (8.314 J/(mol-deg) * 300deg)] * (200/5) μM
Recognizing that 8.314 J/(mol-deg) = 0.008314 kJ/(mol-deg),
[P] = exp[(-27 kJ/mol + 18 kJ/mol) / (0.008314 * 300 kJ/mol)] * 40 μM
[P] = (exp((-9)/(2.4942)) * 40 μM
[P] = exp(-3.608) * 40 μM = 0.0271 * 40 μM = 1.084 μM.
(3 points for correct approach; 2 points for correct answer to within 5%.
As usual, count off one point for wrong or missing units.)